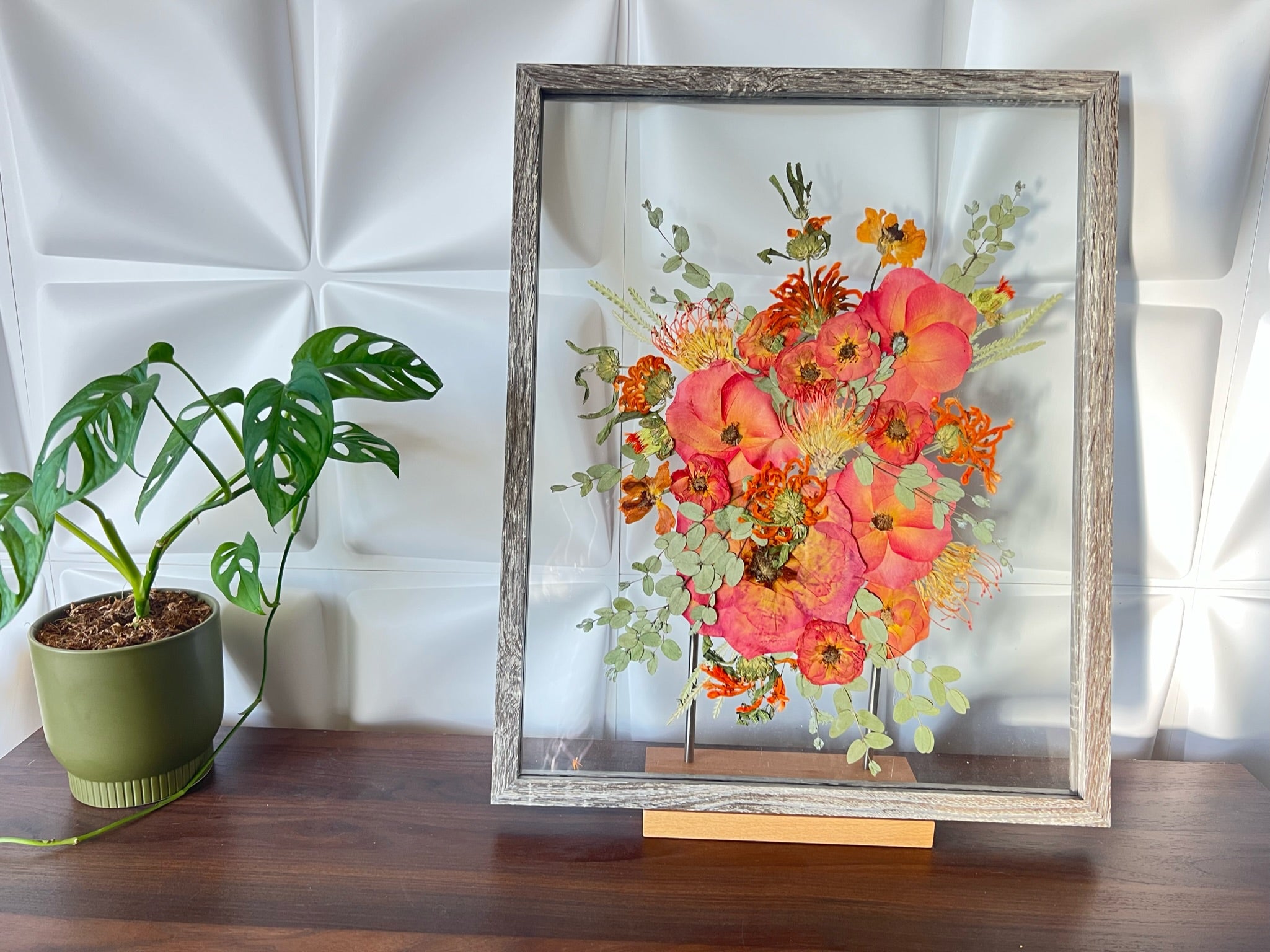Shadow Box With Real Flowers and Photos, Floral Preservation, Pressed & Preserved Flowers in Shadow Box or Float Frame, 3D Flowers - Etsy | Floral preservation, Real flowers, How to preserve flowers

Stained Glass Frame, Pressed Flower Frame, Pressed Plant Frame, Pressed Flowers, Framed Dried Flowers - Etsy UK

Wedding Flower Preservation / Pressed Flower Frames / Framed Flowers / Wedding Flowers / Pressed Bouquets / Pressed Wedding Bouquet - Etsy UK

Pressed Flowers, Large Pressed Flower, 1 St Anniversary, 1 Year Anniversary, Dried Flower Bouquet, Preserve Bouquets, Decoration Mariage - Etsy UK

2 Red Pressed Roses Framed Pressed Rose Flower Wall Art Flower Wall Prints Dried Flower Display Preserved Flower Arrangements - Etsy UK

Custom Floral Preservation, Framed Pressed Flowers, Wedding Flowers Ke – Eight Acorns Floral Preservation

Set of 4 Hanging Double Glass Pressed Flower Brass Frame | Etsy | Pressed flowers, Pressed flower crafts, Pressed flower art

Flower Preservation, Pressed Flower Frame. Wedding Bridal DRIED Flowers, Wedding, Funeral Pressed Flowers, Keepsake - Etsy UK

Flower Preservation. Pressed Flower Frame Wall Hangings. Pressed Flower Frame, Hanging Terrarium, Wedding Funeral Flowers, Glass Frame. - Etsy